Accurately determining cell number is a fundamental technique in various life science disciplines. Whether you’re a researcher studying cell proliferation, a technician analyzing blood samples, or a doctor monitoring cell cultures, the hemocytometer (also spelled hemocytometer) emerges as an essential tool.
What is a Hemocytometer?
A hemocytometer is a specialized microscope slide with a etched grid. The grid design creates a defined counting area, allowing you to calculate the number of cells within a specific volume of your cell suspension. Hemocytometers typically have a double counting chamber, each engraved with a specific grid pattern.
Structure of a Hemocytometer
- Counting Chamber: The chamber has a grid etched into its surface, typically divided into nine large squares, each with a known area.
- Cover Slip: A special thick cover slip is placed over the chamber to create a precise volume.
- Grid Dimensions: The grid lines define areas of 1 mm², and the depth of the chamber is usually 0.1 mm, creating a volume of 0.1 mm³ (1 µL) for each square.
Applications of Hemocytometry
- Cell Culture: Estimating cell concentrations in culture.
- Blood Cell Counting: Determining red and white blood cell counts.
- Microbiology: Counting bacteria or yeast cells.
- Environmental Science: Assessing microbial content in water samples.
How to Use a Hemocytometer
Materials Needed:
- Hemocytometer
- Cover slip
- Pipette
- Cell suspension
- Staining solution (e.g., Trypan Blue for viability staining)
- Microscope
Procedure:
- Preparation:
- Clean the hemocytometer and cover slip with 70% ethanol and dry them.
- Mix the cell suspension thoroughly to ensure even distribution.
- Loading the Sample:
- Pipette a small volume (10-20 µL) of the cell suspension.
- Carefully place the cover slip over the counting chamber.
- Gently load the cell suspension into the chamber by touching the pipette tip to the edge of the cover slip. Capillary action will draw the liquid into the chamber.
- Counting Cells:
- Place the hemocytometer on the microscope stage and focus on the grid.
- Count the cells in the designated squares (usually the four corner squares and the center square of the grid).
How Does it Work?
The hemocytometer counting process involves these key steps:
- Sample Preparation: Dilute your cell suspension to an appropriate concentration that yields a countable number of cells within the hemocytometer grid.
- Filling the Counting Chamber: Using a micropipette, carefully introduce a small volume of the diluted cell suspension onto the hemocytometer platform. Capillary action draws the suspension into the counting chamber.
- Cell Visualization: Place the hemocytometer on a microscope stage and adjust the magnification to visualize the cells clearly within the grid.
- Cell Counting: Systematically count the cells within the defined squares of the grid pattern. Depending on the hemocytometer design, specific counting rules apply (explained in detail below).
Counting Rules and Calculations:
Hemocytometers typically have a central counting area with a grid pattern of 9 large squares, each further subdivided into 16 smaller squares.

Here’s how to calculate cell concentration:
- Choose Counting Squares: Select a defined number of large squares (usually 4 or 5) for counting. Ensure even distribution of cells across the chosen squares.
- Count Cells: Methodically count the number of cells within the small squares of your chosen large squares.
- Calculation:
The following formula allows you to calculate the cell concentration (cells/mL) in your original, undiluted sample:
ell concentration (cells/mL) = (Total Counted Cells) / (Area of Counted Squares x Dilution Factor x Volume Loaded)
xample Calculation:
Let’s assume you counted a total of 80 cells in 4 large squares (each with 16 small squares) of your hemocytometer. You diluted your cell suspension 1:100 before loading 10 μL (0.01 mL) onto the hemocytometer.
Area of Counted Squares: Since each large square has 16 small squares and you counted 4 large squares, the total area of counted squares is 4 squares * 16 squares/square = 64 squares.
Calculation:
Cell concentration (cells/mL) = (80 cells) / (64 squares * 100 dilution factor * 0.01 mL) = 1.25 x 10^4 cells/mL
Therefore, your original, undiluted cell suspension has a concentration of 1.25 x 10^4 cells/mL.
Cell Viability Measurement using Hemocytometer
Trypan blue is a vital stain used to selectively color dead tissues or cells blue. It is a diazo dye. Live cells or tissues with intact cell membranes are not colored Since cells are very selective in the compounds that pass through the membrane, in a viable cell Trypan blue is not absorbed; however, it traverses the membrane in a dead cell. Hence, dead cells are shown as a distinctive blue color under a microscope. Since live cells are excluded from staining, this staining method is also described as a Dye Exclusion Method.
Procedure for calculating cell count and % viability of BHK-21 cell line
- 100uL of the BHK-21 cell suspension was aseptically transferred into an eppendorf tube inside laminar air flow unit.
- Mix the cell suspension with 100 uL of Trypan Blue.
- Fill the haemocytometer chamber with the cell suspension and observe under phase contrast microscope in 10X magnification.
- Count the cells in each of the four grids bordered by triple lines on any two side of the square.
- the percentage of vialbility can be calculated using the formula
% viability = (No of viable cells / Total no of cells) * 100
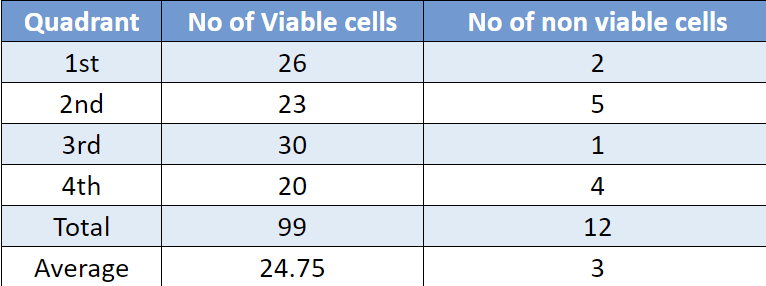
Cell Concentration
Number of cells/ml = Average number of cells/grid x Dilution factor x 10^4.
=24.75 * 2 * 10^4
= 4.9*10^5
% viability = (No of viable cells / Total no of cells) * 100
% viability = (24.75/ 27.75) * 100 = 89.18%
Tips for Accurate Counting
- Homogenize Sample: Ensure the cell suspension is well-mixed to avoid clumping or uneven distribution.
- Avoid Overfilling: Do not overfill the chamber, as this can lead to inaccurate counts.
- Count Multiple Squares: Increase accuracy by counting cells in multiple squares and averaging the results.
- Stain Cells: Use viability stains like Trypan Blue to differentiate between live and dead cells.
Commercial and Clinical Relevance
- Research Laboratories:
- Cell Culture Monitoring: Ensuring optimal cell density for experiments.
- Tissue Engineering: Accurate cell counts are vital for scaffold seeding and growth studies.
- Clinical Diagnostics:
- Hematology: Counting blood cells for diagnosing conditions like anemia or infections.
- Cancer Research: Assessing tumor cell concentrations in samples.
- Biotechnology:
- Bioprocessing: Monitoring microbial populations in fermentation processes.
- Pharmaceuticals: Ensuring cell line consistency for drug production.